Boehringer Ingelheim has posted a 41 rise in 2015 currency-adjusted sales mainly thanks to a rapid acceleration in its diabetes drug revenues. Die Zahl der Diabetes-Erkrankungen wird Schätzungen zufolge bis zum Jahr 2045 auf 629 Millionen steigen.
 Linagliptin 5mg Trajenta For Anti Diabetes Boehringer Ingelheim Rs 490 00 Strip Id 21921369333
Linagliptin 5mg Trajenta For Anti Diabetes Boehringer Ingelheim Rs 490 00 Strip Id 21921369333
Novo Nordisk Sanofi Merck Eli Lilly and Company AstraZeneca Takeda Boehringer Ingelheim Novartis Johnson Johnson Services and Bayer.

Boehringer ingelheim diabetes drugs. In addition to the now marketed products from our own RD and basal insulin from our alliance partner Eli Lilly and Company we are also focused on. Boehringer Ingelheim was founded in 1885 by Albert Boehringer. Jardiance is a sodium glucose co-transporter 2 SGLT2 inhibitor.
German drugmaker Boehringer Ingelheim BI which has cornered about 50 percent market share for its new class of oral anti-diabetic drugs SGLT2 sodium glucose co. Schnell zuverlässig und effizient beliefern wir unsere Kunden und Patienten mit sicheren hochqualitativen Biopharmazeutika über unser globales Produktionsnetzwerk an den Standorten Biberach Deutschland Wien. Boehringer Ingelheim Sued For 23M Over Diabetes Drug IP Law360 London June 17 2020 659 PM BST -- Biopharmaceutical licensing company Royalty Pharma has sued Boehringer Ingelheim in a UK.
Lebanon Beirut April 25th 2016 Boehringer Ingelheim one of the worlds leading pharmaceutical companies held a press conference at Le Vendome Hotel in Beirut to shed light on the latest changes in Cardiovascular CV implications related to Type 2 Diabetes T2D management in coincides with the launch of the latest innovative product Empagliflozin in the country. 1 Typ-2-Diabetes ist eine chronisch progressive Erkrankung. Als forschungsorientiertes Pharmaunternehmen strebt Boehringer Ingelheim danach die Gesundheit von Menschen und Tieren weltweit zu verbessern.
Boehringer Ingelheim inks outcomes-based contract with pharmacy benefit manager for diabetes drug. He was just 24 years old when he hired 28 people to make tartaric acid salts for pharmacies and dyeing works. Reuters - Independent experts on an FDA advisory panel on Wednesday voted against the use of an already approved diabetes FDA panel votes against Lilly-Boehringer Ingelheims diabetes drug TodayHeadline.
Overview of our products. Zu den Langzeit-Komplikationen zählen sowohl Schlaganfall Herzinfarkt und kardiovaskulärer Tod als auch. We see todays meeting as.
The medication is designed to cut the risk of cardiovascular. Boehringer Ingelheim has inked an outcomes-based contract with pharmacy benefit manager Prime Therapeutics for its oral Type II diabetes drug Jardiance. January 29 2018 By Sarah Faulkner.
The US Food and Drug Administration has approved Boehringer Ingelheims Jardiance empagliflozin tablets as an addition to diet and exercise to improve glycemic control in adults with Type 2 diabetes. The company made its first pharmaceutical the analgesic drug Laudanum in 1912. Diabetes Drugs Market Key Players.
Boehringer Ingelheim Pharmaceuticals Inc. Boehringer Ingelheim has proposed a 25 mg dose of empagliflozin for use in type 1 diabetes to help lower the risk of diabetic ketoacidosis an. It works by blocking the reabsorption of glucose blood sugar by the kidney increasing glucose excretion and lowering blood.
Boehringer Ingelheim Pharmaceuticals Inc. BioXcellenceTM ist die Marke mit der Boehringer Ingelheim als zuverlässiger strategischer Herstellungspartner im globalen Auftragskundengeschäft vertreten ist. Weltweit leben circa 425 Millionen Erwachsene mit Diabetes.
Boehringer Ingelheim has proposed a 25 mg dose of empagliflozin for use in type 1 diabetes to help lower the risk of DKA. Type 2 diabetes is a chronic progressive condition and long-term complications include stroke heart attack and cardiovascular death as well as diabetic kidney disease retinopathy arm and leg amputations and autonomic neuropathy. Top-selling drugs include Spiriva Pradaxa and Jardiance.
Typ-2-Diabetes ist mit 90-95 Prozent die häufigste Form.
 Fda Approves Type 2 Diabetes Drug From Boehringer Ingelheim And Lilly
Fda Approves Type 2 Diabetes Drug From Boehringer Ingelheim And Lilly
 Jardiance Empagliflozin For The Treatment Of Type 2 Diabetes Clinical Trials Arena
Jardiance Empagliflozin For The Treatment Of Type 2 Diabetes Clinical Trials Arena
 Fda Blocks Lilly Boehringer S Jardiance From Coveted Type 1 Diabetes Nod Fiercepharma
Fda Blocks Lilly Boehringer S Jardiance From Coveted Type 1 Diabetes Nod Fiercepharma
 Xconomy Fda Rejects Boehringer Ingelheim Eli Lilly Drug For Type 1 Diabetes
Xconomy Fda Rejects Boehringer Ingelheim Eli Lilly Drug For Type 1 Diabetes
 Eli Lilly Spikes After Fda Approves Label Change For Diabetes Drug To Reduce Cardiovascular Death
Eli Lilly Spikes After Fda Approves Label Change For Diabetes Drug To Reduce Cardiovascular Death
Jardiance Is First Diabetes Drug To Improve Cv Outcomes Pmlive
 Jardiance Reduces Total Cardiovascular Events Risk In Diabetic Patients Pharma 기사본문 Kbr
Jardiance Reduces Total Cardiovascular Events Risk In Diabetic Patients Pharma 기사본문 Kbr
 Micardis Boehringer Ingelheim Com
Micardis Boehringer Ingelheim Com
Lilly And Boehringer Ingelheim To Evaluate Diabetes Drug For Treatment Of Heart Failure Pharmafile
 Trajenta Boehringer Ingelheim Com
Trajenta Boehringer Ingelheim Com
 Innovative Diabetes Drug Portfolio Local Partnerships Help Boehringer Ingelheim Take Big Strides In India
Innovative Diabetes Drug Portfolio Local Partnerships Help Boehringer Ingelheim Take Big Strides In India
Boehringer Lilly S Trajenta Wins European Approval Pharmafile
Boehringer Lilly Win Expanded European Licence For Trajenta Pmlive
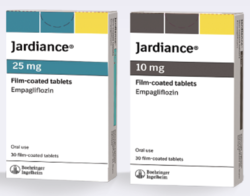
 Jardiance Boehringer Ingelheim Com
Jardiance Boehringer Ingelheim Com
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.