In the future additional devices such as the BD Veritor will be used for rapid antigen screening. The FDA granted an emergency authorization to a portable coronavirus antigen test developed by BD similar to a rapid flu test designed to allow.
 Bd Receives Fda Eua For Combination Antigen Test
Bd Receives Fda Eua For Combination Antigen Test
Organizations must have processes in place to report all positive results from antigen testing to the local public health unit.
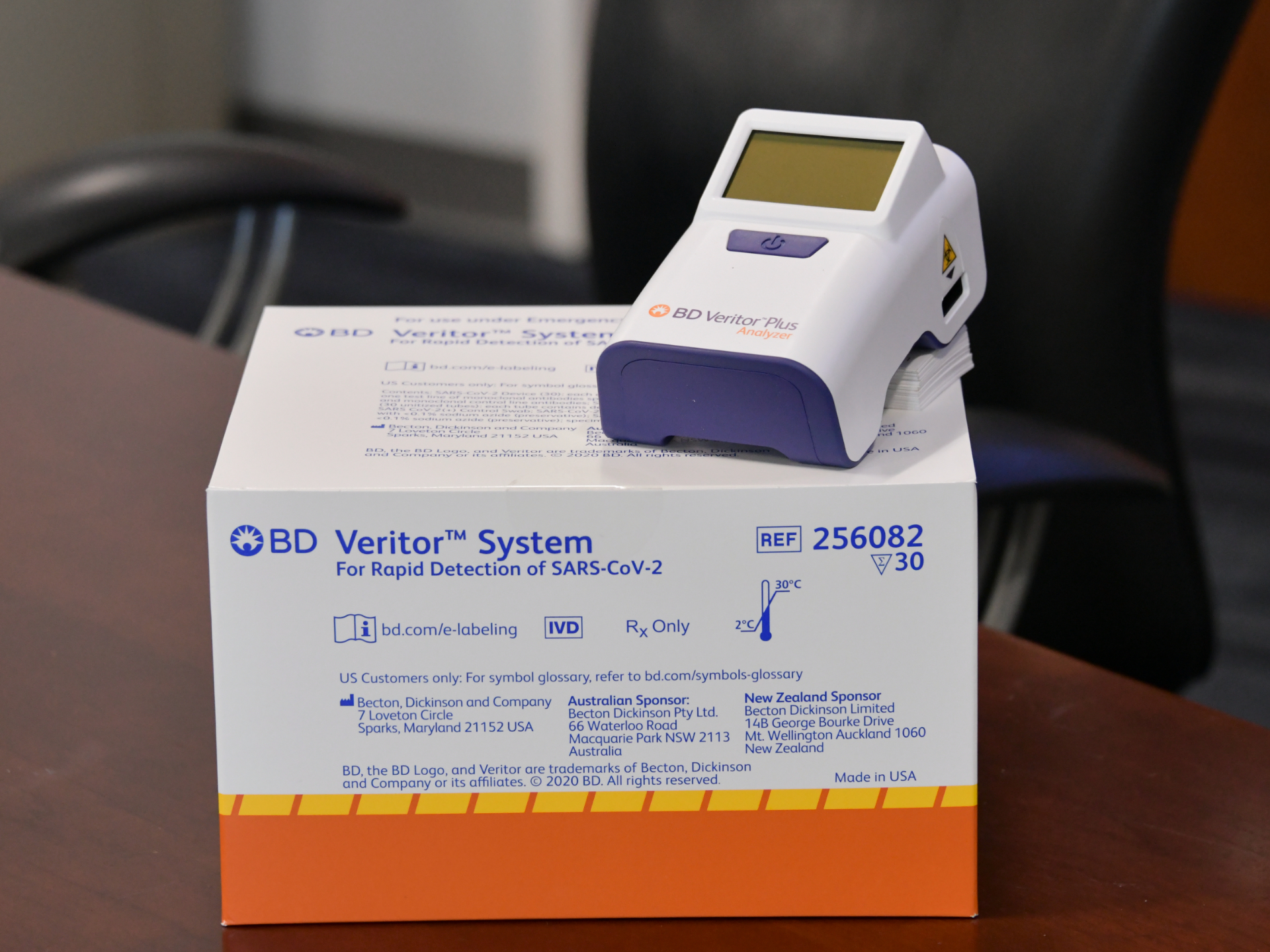
Rapid antigen test from bd. 23 The BD VeritorTM Plus SARS-CoV-2 COVID-19 Antigen Test is intended for use by trained personnel specifically instructed and trained on use of the product. Quidels product SARS Antigen FIA claimed the antigen categorys first EUA in May and delivers results in 15 minutes. BD on Monday announced FDA granted its rapid point-of-care coronavirus antigen test emergency use authorization making it only the second such diagnostic to receive a nod from the regulatory agency.
Reporting app helps facilitate SARS-CoV-2 testing and result reporting from BD Veritor Plus System. 22 2021 PRNewswire -- BD Becton Dickinson and Company NYSE. Becton Dickinson And Company.
BD on Wednesday announced results of a head-to-head study it funded pitting its Veritor antigen test for detection of SARS-CoV-2 against Quidels Sofia assay. Becton Dickinson and Company BDX popularly known as BD received emergency use authorization EUA from the FDA for a new rapid antigen test. BD Announces Collaboration with ImageMover for Rapid Antigen Test Reporting.
24 CV-19 G0113 Abbott Panbio or D Veritor Point of are Testing Pocket Guide March 2 2021 3. Two studies were completed to determine clinical performance. 3 Page.
The COVID-19 Antigen Test Cartridge test is NOT intended to be interpreted visually. FRANKLIN LAKES NJ March 30 2021 PRNewswire -- BD Becton Dickinson and Company NYSE. The BD Veritor System for Rapid Detection of SARSCoV2 antigen test detects proteins from the SARSCoV2 virus.
The BD Veritor Plus System can provide digital diagnostic test results in 15 minutes. Same machine same test same nurse. The sample is prepared added to the assay cartridge incubated and then interpreted by.
BD BDX ImageMover Partner for Rapid Antigen Test Reporting. It found equivalent performance between the two rapid point-of-care diagnostics despite a difference in sensitivity claims published in emergency use authorization documentation that gave the advantage to BDs rival. The company received emergency-use.
Expect More Cash To Head To Shareholders. As California moves away from the color-tiered system of restrictions in June and more COVID-19 vaccines end up in arms businesses event venues and schools are looking to rapid. BD partners with ImageMover for COVID-19 test mobile.
BD BDX Receives FDA EUA for Combination Rapid Antigen Test BD BDX gets FDA EUA for new rapid antigen test which can distinguish between COVID-19. Photo courtesy of BD. In the first study nasal specimens and either.
BDX a leading global medical technology company today. FRANKLIN LAKES NJ March 9 2021 PRNewswire -- BD Becton Dickinson and Company NYSE. Aktien Nachrichten BECTON DICKINSON AKTIE BD Gets Emergency Use Approval For Covid-19 Flu Rapid Antigen Test Push Mitteilungen FN.
A nasal swab is used to collect the specimen from a patient suspected of having COVID19. BDX a leading global medical technology company today announced the US. FRANKLIN LAKES NJ Feb.
Sunday April 18 2021. Rapid antigen test from BD Elon Musk wrote on Twitter likely referring to Becton Dickinsons NYSEBDX rapid antigen test. Food and Drug Administration FDA granted emergency use authorization EUA for a new rapid antigen test that can detect SARS-CoV-2 influenza A and influenza B in a single test.
Musk wrote that the rapid antigen tests hed taken were from BD likely referring to Becton Dickinson and Co. The BD Veritor System for Rapid Detection of SARS-CoV-2 is a chromatographic digital immunoassay intended for the direct and qualitative detection of SARS-CoV-2 nucleocapsid antigens in direct. Prior to initiating screening organizations should make their their local public health unit aware that they will be engaging in rapid screening.
BD Rapid Antigen Test Shows Path Forward. The clinical performance of the BD Veritor System for Rapid Detection of SARS-CoV-2 nucleocapsid antigen Veritor a chromatographic immunoassay used for SARS-CoV-2 point-of-care testing was evaluated using nasal specimens from individuals with COVID-19 symptoms. BDX a leading global medical technology company and Scanwell Health a leader in smartphone-enabled.